Market Overview:
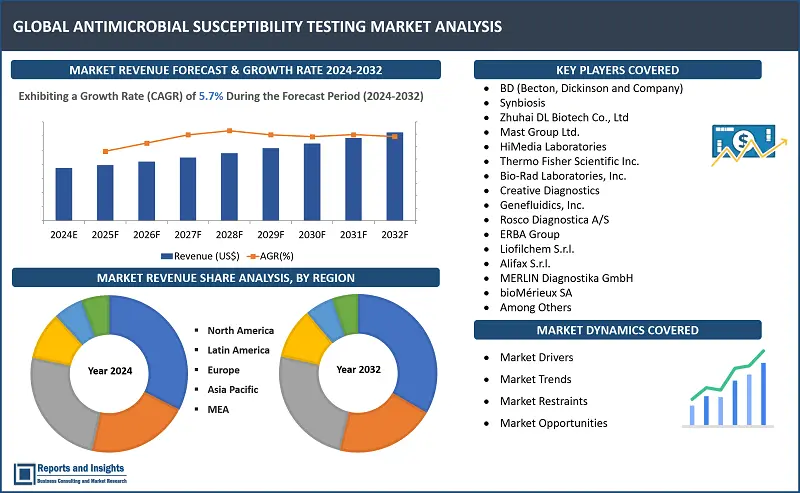
"The antimicrobial susceptibility testing market was valued at US$ 3.7 Billion in 2023, and is expected to register a CAGR of 5.7% over the forecast period and reach US$ 6.1 Bn in 2032."
|
Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Years |
2024-2032 |
|
Historical Years |
2021-2023 |
|
Antimicrobial Susceptibility Testing Market Growth Rate (2024-2032) |
5.7% |
Antimicrobial Susceptibility Testing or AST is a clinical microbiology laboratory test used in the determination of the effectiveness of antimicrobial agents against certain microorganisms, bacteria, or fungi. AST facilitates selection of most appropriate antibiotic treatments for patients with bacterial infections and is employed in the detection of Antimicrobial Resistance (AMR) in patients. Besides, antimicrobial susceptibility testing provides an in vitro measure of bacterial response to an antimicrobial agent that predicts therapeutic efficacy.
The primary step is isolating the microorganism from the patient sample, usually by culturing it on specific media under appropriate conditions, which is then isolated and inoculated onto a solid medium, such as agar, to form a uniform lawn or spread. Antibiotic susceptibility testing involves a number of methods, with two most common methods being disk diffusion method and broth dilution method.
The results of the susceptibility testing are then interpreted based on established breakpoints or guidelines provided by organizations such as the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST). These breakpoints categorize microorganisms as susceptible, intermediate, or resistant to specific antibiotics. The results are reported to the healthcare provider, who then uses this information to guide antibiotic therapy for the patient.
A major and rising concern, is that AMR is a global problem that limits the number of antibiotics that can be used to treat infections. According to estimates by the European Centre for Disease Prevention and Control (ECDC), 30–50% of antimicrobials prescribed to humans are unnecessary, which promotes AMR. Clinical treatment failure is emphasizing the growing role of AST, especially rapid AST (rAST). AST is crucial in monitoring the emergence of resistant pathogens and resistance patterns due to bacterial DNA mutations, and aids in evaluating services.
Major factors driving growth of the Antimicrobial Susceptibility Testing (AST) market are increasing research funding and investments, rising prevalence of infectious diseases, high and rising antibiotic resistance, supportive government initiatives to reduce burden of infectious diseases, high penetration of disc diffusion technique, and developments in susceptibility plates.
Antimicrobial Susceptibility Testing Market Trends and Drivers:
Among some of the key factors driving growth of the global Antimicrobial Susceptibility Testing (AST) market is rising prevalence of infectious diseases worldwide and urgent need for more accurate and timely diagnosis and treatment, which in turn, is driving an incline in antimicrobial susceptibility testing. The other major factor is increasing incidence of antimicrobial resistance raising concerns regarding rising risks of major public health threats.
Also, advancements in technology are certainly aiding in testing methods, and integration for automation and molecular diagnostics are enhancing efficiency, accuracy, and speed or AST. Advancements in medical applications and in guiding antibiotic therapy for bacterial infections, and ensuring patients receive the most effective and appropriate treatment, while also minimizing risk of antibiotic resistance are some key roles technology has been playing. Use of rapid testing methods like molecular diagnostics has been increasing to enable healthcare providers to obtain test results quicker, thereby allowing for prompt adjustments to treatment plans for patients. Point-Of-Care Testing (POCT) devices are facilitating faster decision-making and serving to improve patient outcomes.
Some positive trends in the market include integration of susceptibility testing with Electronic Medical Records (EHRs) and rising focus on developing multiplex testing platforms, strategic collaborations, and acquisitions, which are resulting in companies expanding respective product portfolios and expanding market reach.
Antimicrobial Susceptibility Testing Market Restraining Factors:
Some key factors impacting potential market growth are stringent food safety regulations and need for comprehensive testing for antimicrobial susceptibility, and concerns regarding potential health risks associated with use of antimicrobials such as antibiotic resistance residues, in food production. Also, AST can be costly, and this is more a challenge for smaller food producers or healthcare facilities with limited or constrained budgets. Costs add up due to equipment, reagents, and need for skilled personnel for testing, which has negative impact on wider adoption. In addition, developing accurate and reliable antimicrobial susceptibility testing methods for a broad range of pathogens is complex, and there is also need for such R&D initiatives to constantly evolve to keep up with emerging microbial threats and also to adopt or integrate technological advancements in operations.
Furthermore, while AST may be crucial in identifying effective treatment options and guiding antibiotic stewardship programs, it also has limitations. For one, time required for accurate testing is lengthy, which can delay treatment decisions, and also, potential for false-negative or false-positive results can lead to inappropriate or inaccurate use of antibiotics.
Another major challenge in the market is availability and use of substitutes and alternatives for assessing antimicrobial efficacy, two of which are molecular diagnostic techniques or rapid screening tests for instance, which may have wider appeal due to speed and ease of use.
Antimicrobial Susceptibility Testing Market Opportunities:
Development of innovative testing methods that offer faster turnaround times and greater accuracy: For example, integration of automation and Artificial Intelligence (AI) technologies can streamline the testing process, enabling laboratories to handle higher volumes of samples more efficiently. Also, increasing popularity of point-of-care testing solutions that enable deliverer of rapid results directly at the patient's bedside or in remote settings presents an opportunity for manufacturers to invest in developing and introducing portable and easy-to-use testing devices.
Potential revenue opportunities also present themselves in the form of need for related services, and companies can offer complementary services like consulting and training programs to help healthcare providers to optimize antimicrobial stewardship initiatives. Also, leveraging training and guidance on best practices in antibiotic usage and interpretation of susceptibility test results will serve to empower healthcare providers and staff. This will also enable companies to gain recognition as trusted partners in initiatives against antimicrobial resistance.
Furthermore, entering into strategic partnerships and collaborations with healthcare institutions, research organizations, and regulatory bodies can lead to development of new products and also expand market reach. Additionally, forming joint ventures or acquiring smaller firms specializing in complementary technologies or niche segments in antimicrobial susceptibility testing can help to accelerate product development and innovation.
Antimicrobial Susceptibility Testing Market Segmentation:
By Product Type
- Automated AST Systems
- Manual AST Products
- Consumables
- Antibiotic Discs
- Plates
- Broth
- Accessories
- Inoculating Loops
- Spreaders
- Services and Software
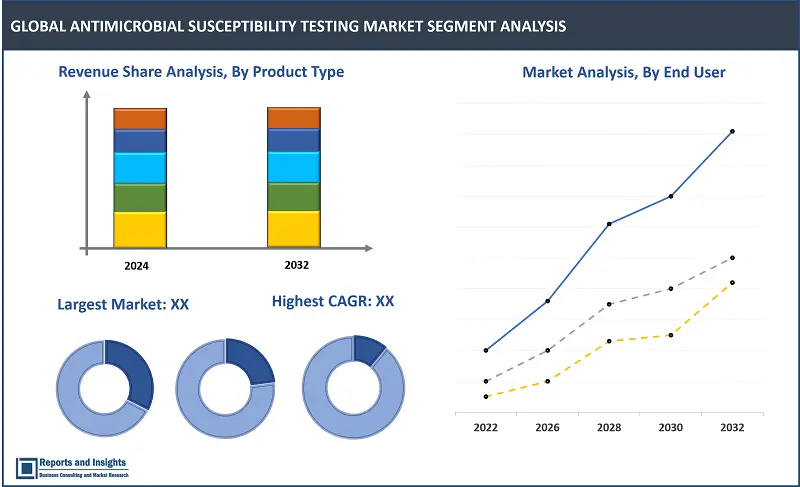
Among the product type segments, the automated AST systems segment is projected to account for the largest revenue share. This is supported by increasing demand for automation in clinical microbiology laboratories to improve testing efficiency, accuracy, and throughput. Automated systems offer streamlined workflows, standardized testing procedures, and integration with laboratory information systems, driving adoption among healthcare facilities worldwide. Also, advancements in technology, such as robotics and AI, further enhance the capabilities and performance of automated AST systems, and this is expected to drive demand and adoption of newer solutions, and support revenue growth of this segment over the forecast period.
By Method
- Disk Diffusion
- Broth Dilution
- E-test
- Molecular Testing
- Polymerase Chain Reaction (PCR)
- Sequencing
Among the method segments, the molecular testing segment, which include PCR and sequencing methods, is expected to account for largest revenue share. This is primarily due to the growing adoption of molecular techniques for their rapid and accurate detection of antimicrobial resistance genes and mutations. Molecular methods offer higher sensitivity, specificity, and multiplexing capabilities compared to traditional methods like disk diffusion and broth dilution. In addition, advancements in molecular diagnostics technologies and rising focus on personalized medicine are factors expected to contribute to projected revenue growth of this segment.
By Application
- Clinical Diagnostics
- Drug Development and Research
- Epidemiology and Surveillance
- Veterinary Diagnostics
Among the application segments, the clinical diagnostics segment is expected to account for largest revenue share, and this can be attributed to rising prevalence of infectious diseases globally, driving demand for accurate and timely diagnosis of microbial infections. Clinical diagnostics constitute a critical aspect of patient care, influencing treatment decisions and antibiotic stewardship efforts. Moreover, the increasing burden of antimicrobial resistance underscores the importance of effective susceptibility testing in clinical settings, and this is further driving adoption and supporting revenue growth of this segment.
By End User
- Hospitals and Clinics
- Diagnostic Laboratories
- Pharmaceutical and Biotechnology Companies
- Research Institutes and Academic Centers
Among the end-user segments, the hospitals and clinics segment is poised to account for largest revenue share. This is primarily due to the high volume of patient samples processed in hospital laboratories and clinics for diagnosing and treating infectious diseases. Also, increasing focus on infection control measures and antimicrobial stewardship programs within healthcare settings further drives the adoption of susceptibility testing technologies. Hospitals and clinics serve as primary points of care, emphasizing the significance of accurate and timely antimicrobial susceptibility testing in patient management.
By Region
North America
- United States
- Canada
Europe
- Germany
- United Kingdom
- France
- Italy
- Spain
- Russia
- Poland
- Benelux
- Nordic
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- Saudi Arabia
- South Africa
- United Arab Emirates
- Israel
- Rest of MEA
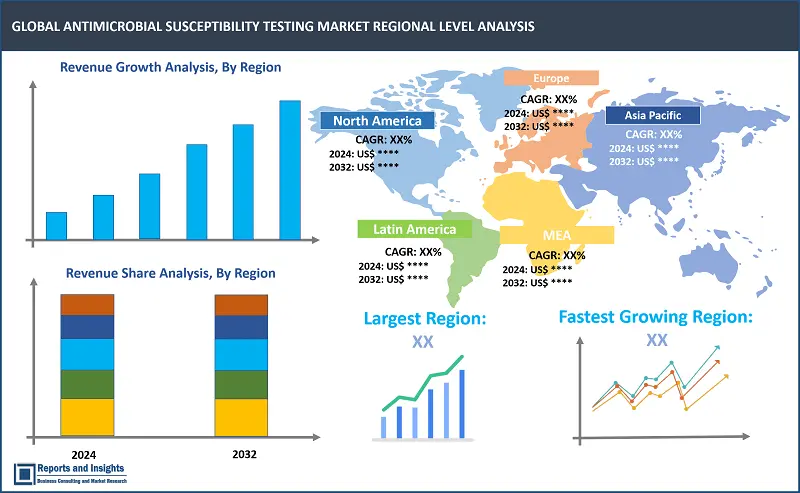
The global antimicrobial susceptibility testing market is divided into five key regions: North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America leads among the regional antimicrobial susceptibility testing markets, with the United States (US) accounting for majority revenue share due to advanced healthcare infrastructure and significant investments in research and development. In Europe, Germany, the United Kingdom (UK), and France are prominent markets, driven by stringent regulatory standards and a high incidence of antibiotic-resistant infections. Asia Pacific market revenue growth rate has been increasing rapidly, with China and India emerging as key markets due to increasing healthcare expenditure, rising awareness about antimicrobial resistance, and expanding clinical research activities.
A prevalent and common factor driving overall growth of the global antimicrobial susceptibility testing market is rising prevalence of infectious diseases, and bacterial and fungal infections worldwide, and need for effective susceptibility testing for appropriate treatment. Also, rising focus on combatting antimicrobial resistance and technological advancements and innovations in automated and molecular testing methods that enhance accuracy, speed, and reliability, are other common driving factors.
Leading Companies in Antimicrobial Susceptibility Testing Market & Competitive Landscape:
The competitive landscape in the global antimicrobial susceptibility testing market is dynamic and characterized by intense competition among key players such as Becton, Dickinson and Company, bioMérieux, Thermo Fisher Scientific, and Danaher Corporation. Leading companies are adopting several strategic initiatives to maintain their market position and expand their consumer base. Some strategies include extensive research and development investments to innovate and enhance testing technologies, resulting in more accurate, rapid, and user-friendly products.
Companies are also focusing on strategic partnerships, mergers, and acquisitions to broaden their product portfolios and geographic reach. In addition, efforts to strengthen distribution networks and provide comprehensive customer support services are crucial in gaining a competitive edge. Emphasis on regulatory compliance and obtaining necessary certifications further establishes trust and credibility among healthcare providers, bolstering market presence. These combined strategies ensure sustained growth and adaptation to evolving market demands in the antimicrobial susceptibility testing sector.
These companies include:
- BD (Becton, Dickinson and Company)
- Synbiosis
- Zhuhai DL Biotech Co., Ltd
- Mast Group Ltd.
- HiMedia Laboratories
- Thermo Fisher Scientific Inc.
- Bio-Rad Laboratories, Inc.
- Creative Diagnostics
- Genefluidics, Inc.
- Rosco Diagnostica A/S
- ERBA Group
- Liofilchem S.r.l.
- Alifax S.r.l.
- MERLIN Gesellschaft für mikrobiologische Diagnostika mbH
- bioMérieux SA
Recent Developments:
- April 2024: QLINEA announced receipt of 510(k) market clearance from the U.S. Food and Drug Administration (FDA) for its ASTar System, paving the way for its introduction to hospitals and laboratories across the United States. Q-linea, known for its innovative infection diagnostics solutions, focuses on creating instruments and disposables for quick and dependable infection diagnostics. The ASTar is a fully automated device designed for Antibiotic Susceptibility Testing (AST), providing a susceptibility profile within six hours directly from a positive blood culture.
- June 2023: Sysmex Corporation, headquartered in Kobe, Japan, introduced a new testing system in Europe, which is designed for the rapid detection of antimicrobial susceptibility. This system, aimed at patients suspected of having Urinary Tract Infections (UTIs), uses urine samples to determine the presence of bacteria and evaluate the effectiveness of antimicrobial treatments. Leveraging proprietary microfluidic technology, the system can deliver Antimicrobial Susceptibility Testing (AST) results in just 30 minutes, significantly faster than the traditional methods that can take several days. This innovation supports the appropriate use of antimicrobial drugs in primary care settings, which are often the initial contact points for patients, and plays a crucial role in combating Antimicrobial Resistance (AMR).
- August 2022: BD (Becton, Dickinson and Company) and Accelerate Diagnostics, Inc. announced a global commercial collaboration. BD will offer Accelerate's rapid testing solutions for antibiotic resistance and susceptibility, delivering results in hours instead of the traditional one to two days. Under this agreement, BD will market the Accelerate Pheno system, Accelerate Arc module, and associated test kits through its global sales network in regions with regulatory approval. These solutions complement BD's Clinical Microbiology portfolio, addressing the global threat of antimicrobial resistance. The Accelerate PhenoTest BC kit, FDA-cleared, provides rapid identification and phenotypic antibiotic susceptibility results directly from positive blood cultures in hours. External studies show it offers results up to two days faster than traditional methods, enabling earlier optimization of antibiotic selection and dosage for patients.
Antimicrobial Susceptibility Testing Market Research Scope
|
Report Metric |
Report Details |
|
Antimicrobial Susceptibility Testing Market size available for the years |
2021-2023 |
|
Base Year |
2023 |
|
Forecast Period |
2024-2032 |
|
Compound Annual Growth Rate (CAGR) |
5.7% |
|
Segment covered |
Product Type, Method, Application, End User, and Region |
|
Regions Covered |
North America: The U.S. & Canada Latin America: Brazil, Mexico, Argentina, & Rest of Latin America Asia Pacific: China, India, Japan, Australia & New Zealand, ASEAN, & Rest of Asia Pacific Europe: Germany, The U.K., France, Spain, Italy, Russia, Poland, BENELUX, NORDIC, & Rest of Europe The Middle East & Africa: Saudi Arabia, United Arab Emirates, South Africa, Egypt, Israel, and Rest of MEA |
|
Fastest Growing Country in Europe |
UK |
|
Largest Market |
North America |
|
Key Players |
BD (Becton, Dickinson and Company), Synbiosis, Zhuhai DL Biotech Co., Ltd, Mast Group Ltd., HiMedia Laboratories, Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Creative Diagnostics, Genefluidics, Inc., Rosco Diagnostica A/S, ERBA Group, Liofilchem S.r.l., Alifax S.r.l., MERLIN Gesellschaft für mikrobiologische Diagnostika mbH, bioMérieux SA, |
Frequently Asked Question
What is the size of the global antimicrobial susceptibility testing market in 2023?
The global antimicrobial susceptibility testing market size reached US$ 3.7 Billion in 2023.
At what CAGR will the global antimicrobial susceptibility testing market expand?
The global market is expected to register a 5.7% CAGR through 2024-2032.
Who is the leader in the antimicrobial susceptibility testing market?
The leader in the antimicrobial susceptibility testing market is Becton, Dickinson and Company, known for its extensive product portfolio and continuous innovation in diagnostic technologies.
What are some key factors driving revenue growth of the antimicrobial susceptibility testing market?
The rising prevalence of infectious diseases, growing threat of antimicrobial resistance, and technological advancements in testing methods.
What are some major challenges faced by companies in the antimicrobial susceptibility testing market?
Major challenges in the market include stringent regulatory requirements, high costs associated with advanced testing technologies, and need for continuous innovation to keep up with evolving pathogens.
How is the competitive landscape in the antimicrobial susceptibility testing market?
The competitive landscape in the antimicrobial susceptibility testing market is highly dynamic and competitive, with key players engaging in extensive R&D, strategic partnerships, mergers, and acquisitions to enhance their market presence.
How is the antimicrobial susceptibility testing market report segmented?
The market report is segmented on the basis of product type, method, application, end user, and region
Who are the key players in the antimicrobial susceptibility testing market report?
Key players in the market report include BD (Becton, Dickinson and Company), Synbiosis, Zhuhai DL Biotech Co., Ltd, Mast Group Ltd., HiMedia Laboratories, Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Creative Diagnostics, Genefluidics, Inc., Rosco Diagnostica A/S, ERBA Group, Liofilchem S.r.l., Alifax S.r.l., MERLIN Gesellschaft für mikrobiologische Diagnostika mbH, and bioMérieux SA

