Market Overview:
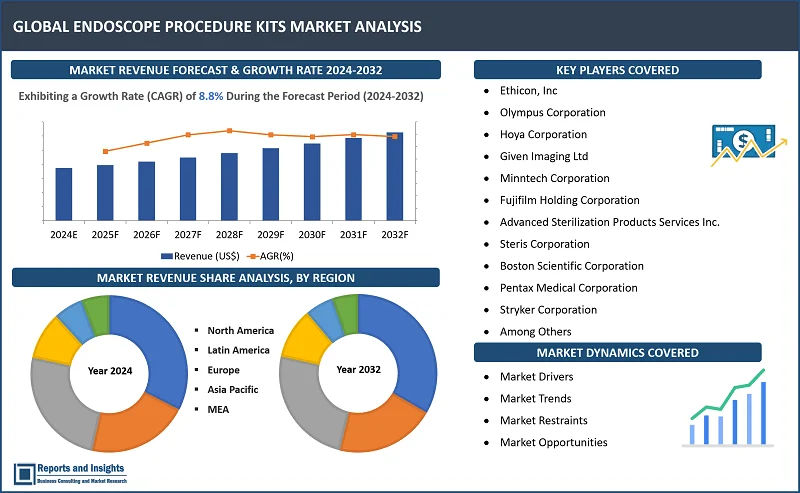
"As per Reports and Insights analysis, the global endoscope procedure kits market was valued at US$ 14.1 Billion in 2023, and is expected to register a CAGR of 8.8% over the forecast period and reach US$ 30.1 Bn in 2032."
|
Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Years |
2024-2032 |
|
Historical Years |
2021-2023 |
|
Market Growth Rate (2024-2032) |
8.8% |
Endoscope procedure kits are specialized collections of medical tools and accessories utilized in endoscopic procedures. These kits typically consist of items like endoscopes (either flexible or rigid tubes with a light and camera at the end), guide wires, forceps, brushes, snares, and cleaning brushes. They are designed to aid in various diagnostic and therapeutic procedures, including examining the gastrointestinal tract, obtaining tissue samples (biopsies), removing polyps, and managing bleeding. These kits are essential for ensuring the safety, efficiency, and efficacy of endoscopic procedures, enabling healthcare professionals to provide optimal care to patients.
The market for endoscope procedure kits is steadily expanding, driven by increasing demand for minimally invasive procedures, advancements in endoscopic technology, and a growing incidence of gastrointestinal disorders. These kits usually consist of various instruments such as biopsy forceps, snares, brushes, and cleaning brushes, as well as accessories like guidewires and catheters. The market is also benefiting from the broader use of endoscopy for therapeutic purposes, in addition to its traditional diagnostic role. Furthermore, there is a noticeable trend toward the use of single-use and disposable kits to reduce infection risks, further propelling market growth.
Endoscope Procedure Kits Market Trends and Drivers:
The endoscope procedure kits market is experiencing growth due to various trends and drivers. These include a growing preference for minimally invasive procedures, advancements in endoscopic technology, and an increasing incidence of gastrointestinal diseases. The market is also benefiting from the expanding elderly population, which is more susceptible to such conditions. Furthermore, the broader application of endoscopy in areas like urology and gynecology is further fueling market growth.
The electric passenger car MRO market growth is influenced by several factors which include increasing incidence of gastrointestinal diseases and the growing demand for minimally invasive diagnostic and therapeutic procedures. Technological advancements in endoscopic equipment, like enhanced imaging and better maneuverability, are also significant drivers. Moreover, the expanding use of endoscopic procedures for both diagnostic and surgical applications, particularly among the elderly population, is contributing to market expansion. Additionally, heightened awareness among patients and healthcare providers about the advantages of early disease detection and treatment is boosting the demand for endoscope procedure kits.
Endoscope Procedure Kits Restraining Factors:
Several factors can impede the growth of the endoscope procedure kits market. These include the high costs associated with endoscopy procedures and equipment, which may restrict access to these kits in certain regions or for patients with limited financial means. Moreover, the complexity of endoscopic procedures and the specialized training required for healthcare providers can hinder the widespread adoption of endoscope procedure kits. Regulatory hurdles and the risk of infection transmission related to inadequately cleaned endoscopes are also significant concerns that can limit market expansion.
Endoscope Procedure Kits Market Opportunities:
The endoscope procedure kits market offers numerous growth opportunities and avenues for innovation. Progress in endoscopic technologies, including the creation of more compact and flexible endoscopes, is broadening the scope of procedures feasible with these kits. A growing incidence of gastrointestinal diseases and cancer is also propelling the need for endoscopic procedures, expanding the market for procedure kits. Additionally, the increasing acceptance of minimally invasive surgeries is anticipated to drive up the demand for endoscope procedure kits due to their benefits, such as shorter recovery periods and lower risk of complications compared to traditional surgical approaches.
Endoscope Procedure Kits Market Segmentation:
By Type
- Bedside Pre-Cleaning Kits
- Transport Pads
- Gauze Pads
- Suction Tubing
- Lubricating Jelly
- Endoscope Valves System
- Pre-cleaning Valve Kit
- Brush Kit
- Others
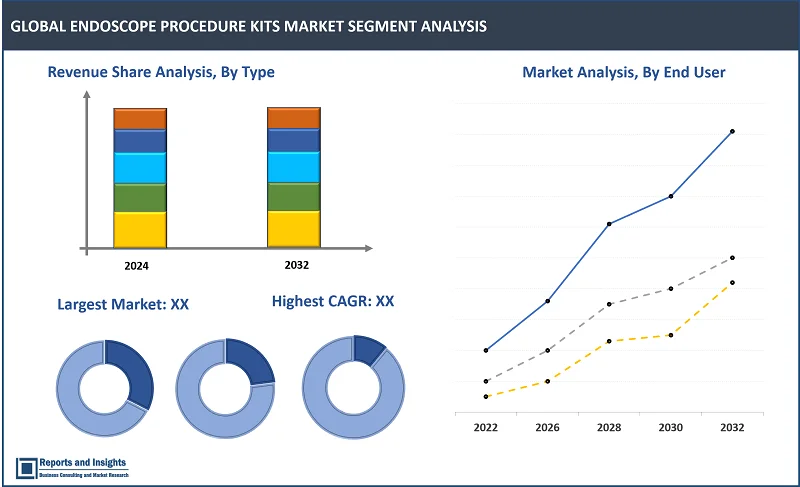
The type segment is further categorised into bedside pre-cleaning kits, transport pads, gauze pads, suction tubing, lubricating jelly, endoscope valves system, pre-cleaning valve kit, brush kit and others. Among these, the bedside pre-cleaning kits are dominating the endoscope procedure market. These kits are crucial for maintaining the cleanliness of endoscopes before procedures, which is vital for preventing infections and ensuring patient safety. While other items like Transport Pads, Gauze Pads, Suction Tubing, and Lubricating Jelly are also important for endoscope procedures, they may not command as significant a market share as the pre-cleaning kits, which are designed specifically for endoscope use.
By Application
- Gastrointestinal Endoscopy
- Bronchoscopy
- Arthroscopy
- Laparoscopy
- Urology
- Others
The application segment is further categorised into gastrointestinal endoscopy, bronchoscopy, arthroscopy, laparoscopy, urology and others. Among these, the gastrointestinal endoscopy is dominating the endoscope procedure market. Gastrointestinal Endoscopy stands out as the leading sub-segment in the endoscope procedure kits market, primarily due to the widespread occurrence of gastrointestinal disorders that necessitate frequent endoscopic procedures for diagnosis and treatment. While Bronchoscopy, Arthroscopy, Laparoscopy, Urology, and other applications also play a role in the market, their contributions are not as significant as that of gastrointestinal endoscopy. Each application area has distinct requirements for endoscope procedure kits, which are tailored to the specific needs of the procedure and the anatomy involved.
By End Users
- Hospitals and Clinics
- Ambulatory Surgical Centers (ASCs)
- Diagnostic Centers
- Others
The end-users’ segment is further categorised into hospitals and clinics, ambulatory surgical centers (ASCs), diagnostic centers and others. Among these, hospitals and clinics are dominating the endoscope procedure market. Hospitals and clinics dominate the endoscope procedure market among end-user segments, primarily due to their capacity to perform a wide array of endoscopic procedures, ranging from routine examinations to complex surgeries. These healthcare facilities are usually well-equipped with advanced endoscopic equipment and accessories and often serve as referral centers for specialized care, contributing to their dominance in the market.
By Region
North America
- United States
- Canada
Europe
- Germany
- United Kingdom
- France
- Italy
- Spain
- Russia
- Poland
- Benelux
- Nordic
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- Saudi Arabia
- South Africa
- United Arab Emirates
- Israel
- Rest of MEA
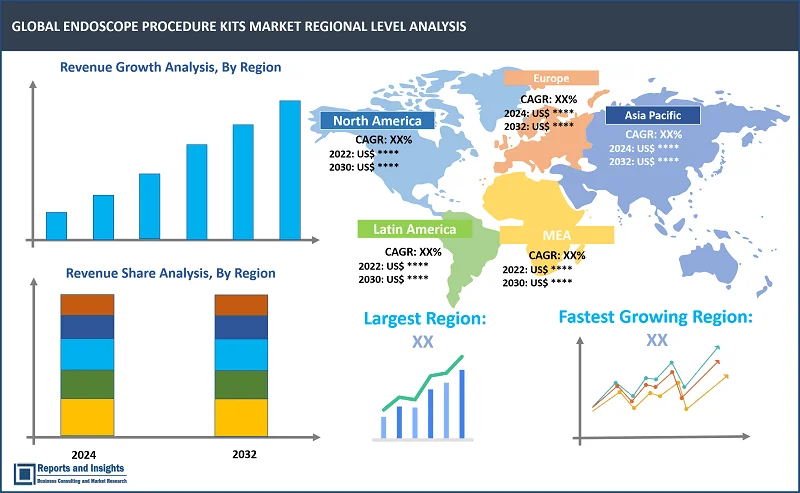
North America leads the endoscope procedure kits market, fueled by factors like a high incidence of gastrointestinal and urological disorders, an aging population, and advanced healthcare facilities. The region's dominance is further supported by the presence of major market players and continuous technological advancements. Europe comes next, with a strong emphasis on medical device research and development and widespread adoption of innovative medical technologies. Meanwhile, Asia-Pacific is an emerging market, experiencing rapid growth due to improving healthcare infrastructure, rising disposable incomes, and increasing awareness of minimally invasive surgical techniques.
Endoscope Procedure Kits Providers & Competitive Landscape:
The endoscope procedure kits market is highly competitive, with several key players vying for market share and actively engaging in strategic initiatives. These companies focus on product innovation, technological advancements, and expanding their product portfolios to gain a competitive edge. These companies are continuously investing in research and development activities to enhance their product offerings and cater to the evolving needs of customers in terms of efficiency, performance, and sustainability.
These companies include:
- Ethicon, Inc
- Olympus Corporation
- Hoya Corporation
- Given Imaging Ltd
- Minntech Corporation
- Fujifilm Holding Corporation
- Advanced Sterilization Products Services Inc.
- Steris Corporation
- Boston Scientific Corporation
- Pentax Medical Corporation
- Stryker Corporation
- KARL STORZ GmBH
- Smith & Nephew plc
- Medtronic plc
- Richard Wolf GmBH
- CONMED Corporation
- Cook Medical Incorporated.
Research Scope
|
Report Metric |
Report Details |
|
Market size available for the years |
2021-2023 |
|
Base Year |
2023 |
|
Forecast Period |
2024-2032 |
|
Compound Annual Growth Rate (CAGR) |
8.8% |
|
Segment covered |
Type, application, end-users and regions. |
|
Regions Covered |
North America: The U.S. & Canada Latin America: Brazil, Mexico, Argentina, & Rest of Latin America Asia Pacific: China, India, Japan, Australia & New Zealand, ASEAN, & Rest of Asia Pacific Europe: Germany, The U.K., France, Spain, Italy, Russia, Poland, BENELUX, NORDIC, & Rest of Europe The Middle East & Africa: Saudi Arabia, United Arab Emirates, South Africa, Egypt, Israel, and Rest of MEA |
|
Fastest Growing Country in Europe |
The U.K. |
|
Largest Market |
North America |
|
Key Players |
Ethicon, Inc., Olympus Corporation, Hoya Corporation, Given Imaging Ltd., Minntech Corporation, Fujifilm Holding Corporation, Advanced Sterilization Products Services Inc., Steris Corporation, Boston Scientific Corporation, Pentax Medical Corporation, Stryker Corporation, KARL STORZ GmBH, Smith & Nephew plc, Medtronic plc, Richard Wolf GmBH, CONMED Corporation and Cook Medical Incorporated. |
Frequently Asked Question
At what CAGR will the endoscope procedure kits market expand?
The market is anticipated to rise at 8.8% through 2032.
What is the market size of the endoscope procedure kits market in 2023?
The endoscope procedure kits market size reached US$ 14.1 Billion in 2023.
What are some key factors driving revenue growth of the endoscope procedure kits market?
Some key factors driving market revenue growth include increasing healthcare expenditure, technological advancements, and aging population.
What are some major challenges faced by companies in the endoscope procedure kits market?
Companies face challenges such as regulatory hurdles, cost constraints, data security and privacy, supply chain challenges, and market fragmentation.
How is the competitive landscape in the endoscope procedure kits market?
Factors such as product quality, reliability, after-sales services, and customization capabilities play a significant role in determining competitiveness.
Who are the leading key players in endoscope procedure kits market?
Ethicon, Inc., Olympus Corporation, Hoya Corporation, Given Imaging Ltd., Minntech Corporation, Fujifilm Holding Corporation, Advanced Sterilization Products Services Inc., Steris Corporation, Boston Scientific Corporation, and others.
What are endoscope procedure kits used for?
Endoscope procedure kits serve a range of purposes, including diagnosing and treating conditions in the gastrointestinal tract, respiratory system, urinary tract, and other internal organs.
Are there any risks associated with endoscope procedure kits?
Endoscope procedure kits are generally safe, with risks such as bleeding, infection, perforation (tearing) of the organ, and adverse reactions to anesthesia or sedation being rare.

