Market Overview:
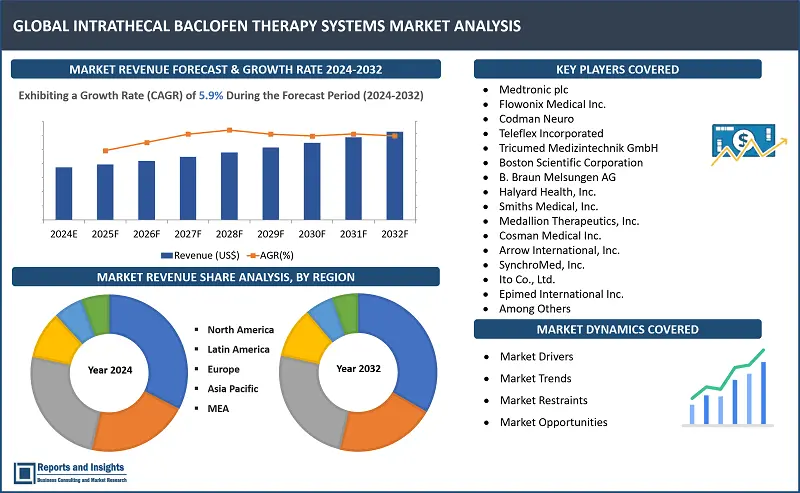
"The intrathecal baclofen therapy systems market was valued at US$ 1.23 Billion in 2023 and is expected to register a CAGR of 5.9% over the forecast period and reach US$ 2.06 Bn in 2032."
|
Report Attributes |
Details |
|
Base Year |
2023 |
|
Forecast Years |
2024-2032 |
|
Historical Years |
2021-2023 |
|
Intrathecal Baclofen Therapy Systems Market Growth Rate (2024-2032) |
5.9% |
An Intrathecal Baclofen (ITB) therapy system typically comprises a programmable pump, an intrathecal catheter, and external programmer to provide specialized medical treatment by delivering medication directly to the area of the spinal canal to manage chronic pain and spasticity when other oral medication options have proven unsuccessful. Baclofen is considered the most-effective drug to treat spasticity, and is a specific agonist of gamma-aminobutyric acid-B receptors. GABA-B receptors are abundant in the superficial layers of the spinal cord, and when administered orally, does not easily penetrate the blood-barrier, and is distributed equally to the brain and spinal cord. However, when baclofen is intrathecally administered, it results in a greater than four-fold increase in Cerebra Spinal Fluid (CSF) level with 1% of the oral dose.
Intrathecal Baclofen (ITB) therapy involves a pump being surgically implanted under the skin in the abdominal or buttocks area of patient, to which a catheter is connected and extends to the spinal cord. Baclofen is in a class of medications known as skeletal muscle relaxants, and ITB therapy is used to directly control spasticity in the spinal cord with fewer systemic adverse effects in a variety of neurological diseases including spinal cord injury, CP, stroke, traumatic brain injury, and hypoxic brain injury. Baclofen is a muscle relaxer and does not treat pain in the way as narcotic medication, but helps to relaxing certain muscles in the patient’s body, relieving spasms, cramping, and tightness of muscles caused by medical conditions such as Cerebral Palsy (CP), Multiple Sclerosis (MS), and certain spinal injuries.
In addition, ITB is an effective treatment for refractory hypertonia in children and besides being used for treatment of spasticity, indications have evolved to include dystonia and mixed Pediatric Movement Disorders (PMDs). ITB is typically used to provide children with spasticity, MS, or other conditions that cause spastic or tight muscles, potent, long-lasting relief from symptoms. Moreover, intrathecal baclofen is widely used in the treatment of patients with intractable spasticity caused by spinal cord injury with severe and disabling autonomic dysreflexia and orthostatic hypotension.
Intrathecal Baclofen Therapy Systems Market Trends and Drivers:
ITB enables effective targeted baclofen delivery for long-term pain management and treatment, and the need for this approach is increasing due to rising prevalence of chronic pain and spasticity conditions, multiple sclerosis, and severe chronic back pain in individuals of varying ages. In addition, increasing shift towards intrathecal therapy systems and Intrathecal Drug Delivery (IDD) systems as a result of technological advancements enhancing safety and efficacy are driving adoption and use. Moreover, device enhancements, including programmable pumps for personalized dosing, are opening up avenues for integration of more innovative approaches for treatment. This includes remote monitoring capabilities as well as positive patient outcomes, which are key factors expected to continue to support growth of the market.
Rising awareness regarding new and emerging technologies and devices, integration with advanced technologies in medicine, and availability of more advanced management solutions in intrathecal therapy for treating various conditions and diseases is supporting market growth. Furthermore, clinical trials demonstrating the efficacy and safety of this approach for various conditions have been positive, and have been serving to encourage physician and patient confidence. Product strategies with regard to miniaturization and patient-centric designs are also playing key roles in further advancements in the medical field and applications.
Another major factor supporting growth of the market is rising healthcare expenditure and greater access to advanced medical treatments in emerging markets. Also, awareness regarding spasticity management and availability of minimally-invasive surgical options for treating associated conditions is having a positive impact on adoption of these systems and procedures. Technological advancements are enabling development of smaller, and more efficient pumps, improved refill procedures and approaches, and allowing for better addressing of patient and clinician needs. Increasing clinical evidence to support long-term benefits of ITB therapy in supporting mobility and reducing pain in patients with severe spasticity is also serving to encourage supportive healthcare policies and reimbursement in various regions and countries.
Intrathecal Baclofen Therapy Systems Market Restraining Factors:
A primary factor having a negative impact on further potential growth of the market is high cost of overall ITB therapy. The costs include products and surgical implantation of pump, as well as costs for continuous maintenance. This can be a challenge for patients as well as healthcare systems, and is especially applicable in developing economies.
Also, stringent drug safety regulations and potential safety concerns, related to risks of infection, device/pump malfunction, or dislodgement of catheter can cause certain level of hesitancy among patient and healthcare providers. In addition, challenges related to Research and Development (R&D), including complexity in designing durable, efficient, and reliable ITB systems, adds up to the already high cost and this is slowing down potential innovation in the market.
Presence of effective substitutes and alternative treatments, such as oral medications and therapies, can have some level of impact on demand for ITB therapy and related solutions. Such alternatives also present fewer risks, and costs are comparatively lower, there positioning these well for a sizable number of patients. Moreover, disadvantages of this therapy, such as potential complications due to surgery and need for regular follow-ups and pump refills can pose some level of restraint to preference among some patients.
Furthermore, unfavorable healthcare reimbursement policies in some regions can limit affordability and preference among a significantly large patient pool, especially as it is complex to obtain medical insurance cover for some expensive treatments.
Intrathecal Baclofen Therapy Systems Market Opportunities:
Investing in development of next-generation ITB systems with enhanced features, improved battery life, advanced programming, and remote monitoring capabilities can open up avenues for manufacturers to broaden patient base and expand market share.
Also, expanding into emerging markets such as Asia Pacific and Latin America can present significant growth opportunities owing to increased healthcare spending and rising prevalence of neurological disorders, as well as unmet needs related to CP and MS. Entering into strategic partnerships with domestic healthcare providers and distributors can facilitate easy market entry and regional expansion, and wider reach of offerings. Also, companies can expand revenue potential by diversifying product portfolios, and developing complementary products such as refill kits, diagnostic tools, and smartphone apps to facilitate patient monitoring can enhance overall treatment experience for patients and healthcare providers.
In addition, entering into strategic agreements, mergers and acquisitions, and contracts can accelerate market penetration. Collaborations with technology firms and research institutions, as well as acquiring smaller companies with innovative technologies can enhance product offerings and add a competitive edge, besides opening up access to new markets and customer segments.
Intrathecal Baclofen (ITB) Therapy Systems Market Segmentation:
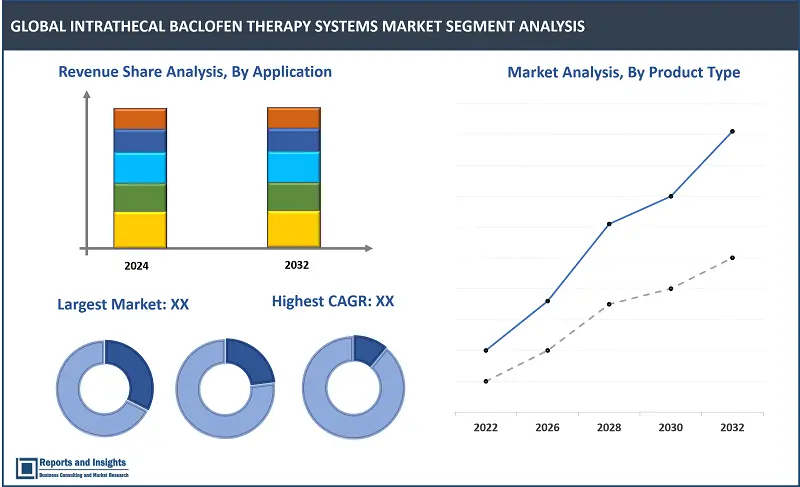
By Product Type
- Programmable Pumps
- Non-programmable Pumps
Among the product type segments, the programmable pumps segment is expected to account for the largest revenue share in the Intrathecal Baclofen (ITB) therapy systems market. This projection is supported by increasing use owing to advanced features, such as precise dosage control and features to adjust delivery rates according to patient needs. Also, the flexibility and customization offered by programmable pumps make this type highly preferable for managing complex spasticity conditions, and this is driving higher adoption compared to non-programmable pumps.
By Application
- Multiple Sclerosis (MS)
- Cerebral Palsy (CP)
- Spinal Cord Injury
- Brain Injury
- Others
Among the application segments, the Multiple Sclerosis (MS) segment is expected to account for the largest revenue share. This projection is supported by the high prevalence of MS worldwide and significant need for effective spasticity management in these patients. ITB therapy provides targeted and sustained relief from severe spasticity associated with MS, improving mobility and quality of life. Also, rising awareness and diagnosis of MS, along with advancements in ITB technology, are expected to further drive adoption and support revenue growth of this segment.
By End User
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
- Homecare Settings
Among the end user segments, the hospitals segment is expected to account for the largest revenue share over the forecast period. This can be attributed to the availability of advanced medical infrastructure and specialized healthcare professionals capable of performing the complex ITB implantation procedure in these settings. Also, hospitals serve as primary centers for diagnosis, treatment, and follow-up care for severe spasticity conditions. The comprehensive care and post-operative support provided in hospital settings by default results in higher adoption and administering of various treatments and services.
By Pump Type
- Constant Rate Pumps
- Variable Rate Pumps
The variable rate pumps segment is expected to account for the largest revenue share over the forecast period. This is attributable to higher preference and use owing to variable rate pumps providing greater flexibility and precision in drug delivery, allowing for adjustments based on individual patient needs and varying levels of spasticity throughout the day. Ability of variable rate pumps to cater to customized treatment regimens improves patient outcomes and satisfaction. The advanced technology and increased efficacy associated with variable rate pumps is also driving appeal, resulting in higher adoption rates.
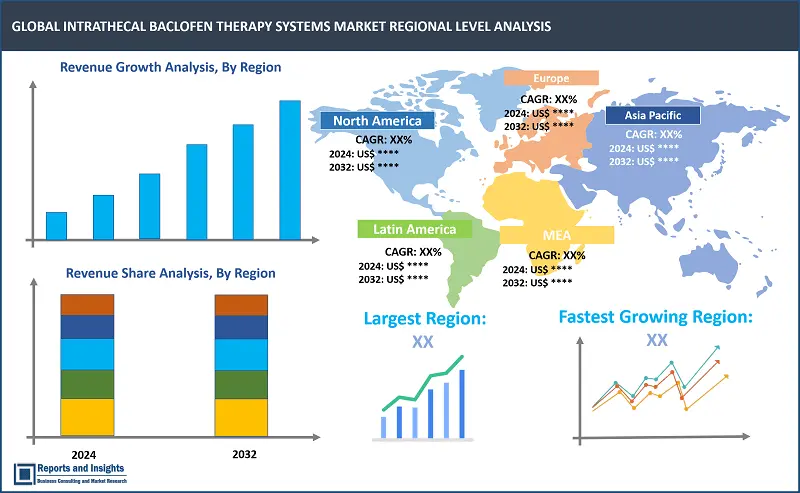
By Region
North America
- United States
- Canada
Europe
- Germany
- United Kingdom
- France
- Italy
- Spain
- Russia
- Poland
- Benelux
- Nordic
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- Saudi Arabia
- South Africa
- United Arab Emirates
- Israel
- Rest of MEA
The global Intrathecal Baclofen (ITB) therapy systems market is divided into five key regions: North America emerges as the leading regional market, driven by advanced healthcare infrastructure and higher adoption rates of innovative medical technologies. The United States (US) leads in North America in terms of revenue share, owing to presence of robust healthcare system and extensive research activities.
In Europe, countries such as Germany and the United Kingdom (UK) lead in the market due to strong healthcare frameworks and increased patient awareness. Asia Pacific market is registering steady revenue growth, with China and Japan contributing significantly as a result of increased healthcare investments and rising prevalence of neurological disorders. Some common factors driving overall growth of the regional ITB therapy systems markets include increasing prevalence of neurological disorders requiring spasticity management, technological advancements in pump design and programming capabilities, and expanding awareness among healthcare professionals and patients about the benefits of ITB therapy.
Leading Companies in Intrathecal Baclofen Therapy Systems Market & Competitive Landscape:
The competitive landscape in the global Intrathecal Baclofen (ITB) therapy systems market is characterized by presence of a number of key players competing to maintain positions and expand respective consumer bases. Leading companies are adopting various strategies to achieve these objectives, with continuous innovation in product development, focus on enhancing pump design, programming capabilities, and patient monitoring features to improve treatment outcomes and patient experience being some common approaches. In addition, strategic collaborations with healthcare institutions and research organizations enable companies to stay in the know about market trends and emerging technologies, ensuring their offerings remain competitive.
Furthermore, aggressive marketing efforts and educational initiatives aimed at raising awareness about ITB therapy among healthcare professionals and patients are crucial for expanding consumer bases. These strategies collectively contribute to the dynamic competitive landscape of the ITB therapy systems market.
These companies include:
- Medtronic plc
- Flowonix Medical Inc.
- Codman Neuro
- Teleflex Incorporated
- Tricumed Medizintechnik GmbH
- Boston Scientific Corporation
- B. Braun Melsungen AG
- Halyard Health, Inc.
- Smiths Medical, Inc.
- Medallion Therapeutics, Inc.
- Cosman Medical Inc.
- Arrow International, Inc.
- SynchroMed, Inc.
- Ito Co., Ltd.
- Epimed International Inc.
Recent Developments:
- October 2023: Medtronics received approval of its next-generation SynchroMed III intrathecal drug delivery system for those patients suffering from chronic pain, severe spasticity and cancer pain. SynchroMed III is a targeted drug delivery deliver edication directly to the fluid surrounding the spinal cord.
- June 2022: Amneal Pharmaceuticals launched a new medication with name, LYVISPAH. This is a specialty drug approved by the FDA to treat muscle stiffness caused by multiple sclerosis and other spinal cord conditions.
Intrathecal Baclofen (ITB) Therapy Systems Market Research Scope
|
Report Metric |
Report Details |
|
Intrathecal Baclofen Therapy Systems Market Size available for the years |
2021-2023 |
|
Base Year |
2023 |
|
Forecast Period |
2024-2032 |
|
Compound Annual Growth Rate (CAGR) |
5.9% |
|
Segment covered |
Product Type, Application, End User, Pump Type |
|
Regions Covered |
North America: The U.S. & Canada Latin America: Brazil, Mexico, Argentina, & Rest of Latin America Asia Pacific: China, India, Japan, Australia & New Zealand, ASEAN, & Rest of Asia Pacific Europe: Germany, The U.K., France, Spain, Italy, Russia, Poland, BENELUX, NORDIC, & Rest of Europe The Middle East & Africa: Saudi Arabia, United Arab Emirates, South Africa, Egypt, Israel, and Rest of MEA |
|
Fastest Growing Country in Europe |
UK |
|
Largest Market |
North America |
|
Key Players |
Medtronic plc, Flowonix Medical Inc., Codman Neuro, Teleflex Incorporated, Tricumed Medizintechnik GmbH, Boston Scientific Corporation, B. Braun Melsungen AG, Halyard Health, Inc., Smiths Medical, Inc., Medallion Therapeutics, Inc., Cosman Medical Inc., Arrow International, Inc., SynchroMed, Inc., Ito Co., Ltd., Epimed International Inc. |
Frequently Asked Question
What is the size of the global Intrathecal Baclofen (ITB) therapy systems market in 2023?
The global Intrathecal Baclofen (ITB) Therapy Systems Market size reached US$ 1.23 Billion in 2023.
At what CAGR will the global Intrathecal Baclofen (ITB) therapy systems market expand?
The global market is expected to register a 5.9% CAGR through 2024-2032.
Who are leaders in the Intrathecal Baclofen (ITB) therapy systems market?
Medtronic plc, Flowonix Medical Inc., and Codman Neuro are known for innovative ITB therapy systems and extensive market reach.
What are some key factors driving revenue growth of the Intrathecal Baclofen (ITB) therapy systems market?
Some key factors driving revenue growth of the market include the rising prevalence of neurological disorders requiring spasticity management, technological advancements in pump design and programming capabilities, and increasing awareness among healthcare professionals and patients about the benefits of ITB therapy.
What are some major challenges faced by companies in the Intrathecal Baclofen (ITB) therapy systems market?
Major challenges faced by companies in the market include stringent regulatory requirements, high treatment costs, potential surgical complications, and competition from alternative treatment options such as oral medications and physical therapy.
How is the competitive landscape in the Intrathecal Baclofen (ITB) therapy systems market?
The competitive landscape in the market is characterized by presence of key players such as Medtronic plc, Flowonix Medical Inc., and Codman Neuro, along with several smaller companies. These companies compete based on factors such as product innovation, pricing strategies, geographic presence, and distribution networks.
How is the Intrathecal Baclofen (ITB) therapy systems market report segmented?
The Intrathecal Baclofen (ITB) therapy systems market report is segmented based on product type, application, end user, pump type, and region.
Who are the key players in the Intrathecal Baclofen (ITB) therapy systems market report?
Medtronic plc, Flowonix Medical Inc., Boston Scientific Corporation, Codman Neuro, Teleflex Incorporated, Tricumed Medizintechnik GmbH, Boston Scientific Corporation, B. Braun Melsungen AG, Halyard Health, Inc., Smiths Medical, Inc., Medallion Therapeutics, Inc., and among others.

